Unlock The Power Of Clean Data
Share Now:
Client
A rapidly growing niche pharmaceutical company developing innovative treatments based in the US.
Industry Challenge
Our client was facing a significant hurdle in their clinical trial process – ensuring the accuracy and traceability of their data. Manual data collection methods as well as usage of a variety of different methods and tools were prone to errors, and inconsistent data formats across various clinical location created challenges in data analysis. This raised concerns about regulatory compliance and the overall integrity of their clinical trials.
Solution
Our team of data integrity experts collaborated with our client to develop a comprehensive solution. This included:
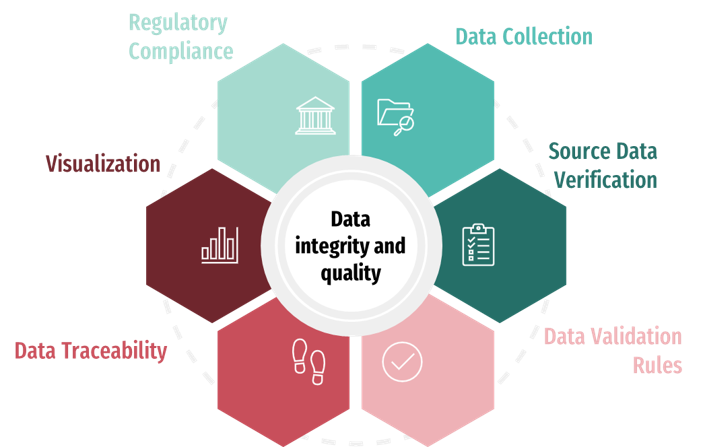
- Standardized Data Collection Tools: We implemented a cloud-based electronic data capture (EDC) system. This standardized platform ensured consistent data format across all clinical sites, minimizing errors and streamlining data collection.
- Automated Data Validation Rules: We configured automated data validation rules within the EDC system. These rules flagged inconsistencies and potential errors in real-time, allowing for immediate correction and improved data quality.
- Enhanced Audit Trails: We established a robust audit trail system, documenting every step of the data collection and management process. This enhanced transparency and traceability, fostering trust and regulatory compliance.
Data Integrity & Quality Framework
Transformative Impact
By implementing these solutions, the client achieved significant improvements in their clinical trial data integrity:
- Reduced Data Errors: The automated validation rules significantly reduced data entry errors, leading to cleaner and more reliable data sets.
- Improved Data Analysis Efficiency: Standardized data formats facilitated faster and more efficient data analysis, accelerating the clinical trial process.
- Enhanced Audit Trails: We established a robust audit trail system, documenting every step of the data collection and management process. This enhanced transparency and traceability, fostering trust and regulatory compliance.
- Increased Confidence in Results: With improved data quality, the client gained greater confidence in the validity and reliability of their clinical trial results.
